An analysis of eight European studies into the efficacy of Soratinex (2001 to 2016)
TOTAL PATIENTS: 2050
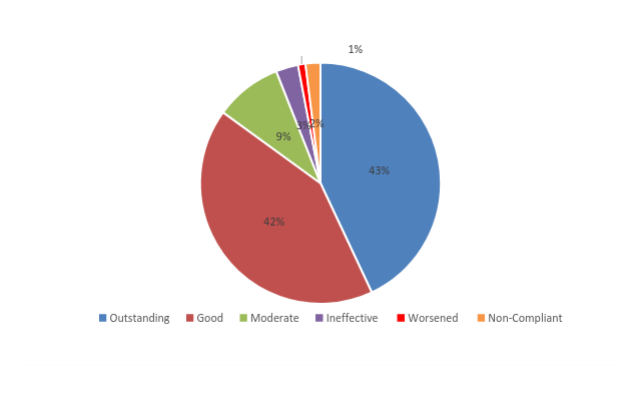
- 46 non-compliant and discontinued treatment (2%)
Of those who continued treatment
- Outstanding improvement (76-100%) in 881 patients (43%)
- Good improvement (51-75%) in 852 patients (42%)
- Moderate improvement (26-50%) in 184 patients (9%)
- Ineffective (0-25%) in 59 patients (3%)
- 28 patients worsened and discontinued (1%)
(Source: Journal of Biological Regulators & Homeostatic Agents, April-June 2016. Analysis: FRANKL Pharma. All studies took place between 2001 and 2016).
https://www.ncbi.nlm.nih.gov/pubmed/27498651 - A multi-centred open trial of “Dr Michaels®” (also branded as Soratinex®) topical product family in psoriasis.
https://www.ncbi.nlm.nih.gov/pubmed/27498668 - Efficacy and safety of Dr Michaels® (Soratinex®) product family for the topical treatment of psoriasis: a monitored status study.
https://www.ncbi.nlm.nih.gov/pubmed/27498655 - Clinical evaluation of the effectiveness of “Dr Michaels®” (also branded as Soratinex®) products in the topical treatment of patients with plaque psoriasis.
https://www.ncbi.nlm.nih.gov/pubmed/27498669 - Quality of life aspects of patients with psoriasis using a series of herbal products.
https://www.ncbi.nlm.nih.gov/pubmed/27498667 - An innovative, promising topical treatment for psoriasis: a Romanian clinical study.
https://www.ncbi.nlm.nih.gov/pubmed/27498666 - Scalp psoriasis: a promising natural treatment.
https://www.ncbi.nlm.nih.gov/pubmed/27498657 - Dr Michaels® (Soratinex®) product for the topical treatment of psoriasis: a Hungarian/Czech and Slovak study.
https://www.ncbi.nlm.nih.gov/pubmed/27498653 - A clinical examination of the efficacy of preparation of Dr Michaels® (also branded as Soratinex®) products in the treatment of psoriasis.
